Why Is Blind Testing Used In The Drug Approval Process
A Short History By Suzanne White Junod PhD. 1 The function of the controlled clinical trial is not the discovery of a new drug or therapy.

Fda Update The Fda S New Drug Approval Process Development Premarket Applications
Three stages of testing drugs.

Why is blind testing used in the drug approval process. The current system for developing testing and regulating vaccines developed during the 20 th century as the groups involved standardized their procedures and regulations. THE DRUG DISCOVERY PHASE Drug discovery is a process which aims at identifying a compound therapeutically useful in treating and curing a disease. Good blinding can reduce or eliminate experimental biases that arise from a participants expectations observers effect on the participants observer bias confirmation bias and other sources. Clinical trials test potential treatments in human volunteers to see whether they should be approved for wider use in the general population. In a blind or blinded experiment information which may influence the participants of the experiment is withheld until after the experiment is complete. Both of these kinds of testing are however safe and not intrusive for the participants because of the Institutional Review Boards IRBs and debriefings that.
The standard for testing and monitoring of vaccines is higher than it is for most other medicines. The Drug Development and Approval Process. During the six to seven years of preclinical testing the. Clinical trialsare studies to test new drugs already approved drugs devices or other forms of treatments. The drugs are tested using computer models and human cells grown in the laboratory. A treatment could be a.
The FDA estimates that it takes a little over 8 years to test a drug including early laboratory and animal testing before there is final approval for use by patients. How vaccines are tested Understandably people are often concerned to know how rigorously and extensively vaccines have been tested. Because blind and double-blind testing involves experimentation on participants who are unaware of their own exposer to an independent variable they can lead people to worry about the safety of the participants. For example willow bark was used. Early patient studies tolerance kinetics pharmacology efficacy proof of concept dose range drug interactions special patient populations. Drug companies continuously analyze thousands of compounds seeking ones of therapeutic value.
A blind sample testing is a drug screen procedure that involves the collection submission and confirmation of an artificial urine specimen to ensure laboratory facilities meet test result standards mandated by the Department of Transportation DOT. Certain drugs can be extracted from natural sources and have been known about for a long time. FDA and Clinical Drug Trials. Researchers still use human volunteers to test these methods and the same rules. The FDAs decision whether to approve a new drug for marketing comes down to answering two. Federal funding of grants and contracts to perform clinical trials of orphan products 2.
This is especially true for new vaccines. There are three main stages of testing. Data for registration via double-blind trials against competitors on efficacy and safety. 50 Tax Credit for costs of clinical testing 3. Exclusive right to market the drug for 7 years from the marketing approval date. Some even look at ways to prevent diseases from happening.
This page aims to outline the process involved in developing and licensing a vaccine for use in the UK. Controlled clinical trials in which results observed in patients getting the drug are compared to the results observed in patients receiving a different treatment are the best way science has come up with to determine what a new drug really does. The process of drug discovery involves the identification of the candidates Synthesis characterisation screening and assays for therapeutic efficacy. About 110 vaccines are in development as of mid-2020. Usingblind and double blind trials make it so there is not any biastowards a certain drug. Also since only 69 of medications undergoing Phase II trials meet their TPPs Table 1 and only about 10 of new drugs eventually gain FDA approval the first successful SARS-CoV-2 vaccine will most likely be the 42nd or even the 90th one to complete human testing.
Designed to promote the development and manufacture of orphan drugs those drugs used in the treatment of rare diseases 1. Vaccine development is a long complex process often lasting 10-15 years and involving a combination of public and private involvement. A blind can be imposed on. The manual inspection process employs the use of a lighted booth Figure 15-1 with a back wall composed of non-glare material that is. With manual inspection an inspector physically picks up and examines the drug product containers. The process of developing and testing a new drug is a lengthy one.
The process of getting a drug to market from first testing to final FDA approval is summarized in figure 1 and described at greater length below. A laboratory can receive up to a maximum of fifty blind samples on a quarterly basis with the proportion of the. Those are used to get the most correct results possible. Resulting in correct trials. Good Laboratory Practice GLP Toxicology Phase. Many clinical trials look at new ways to detect diagnose or measure the extent of disease.
Candidate drug CD prenomination. The Drug Development and Approval Process.
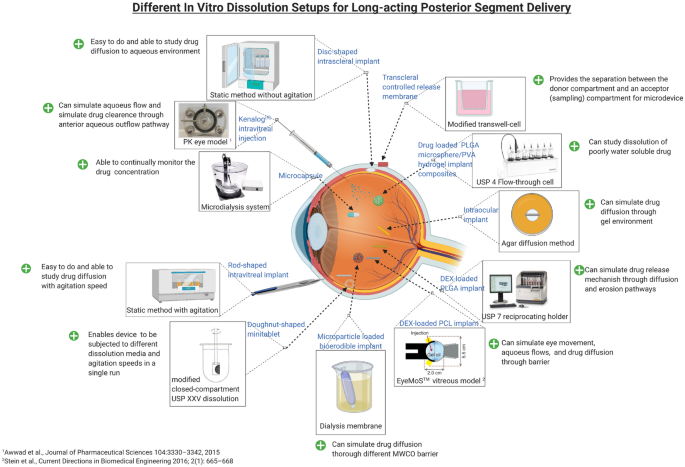
In Vitro Dissolution Testing Models Of Ocular Implants For Posterior Segment Drug Delivery Springerlink
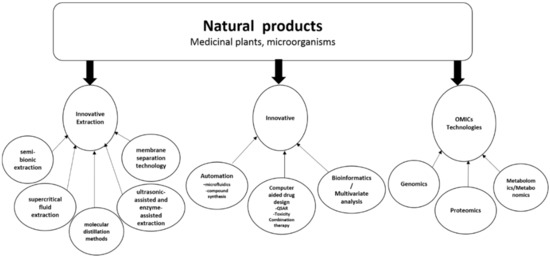
Ijms Free Full Text Natural Products For Drug Discovery In The 21st Century Innovations For Novel Drug Discovery Html
Regardd Regulatory Guidance For Academic Research Of Drugs And Devices
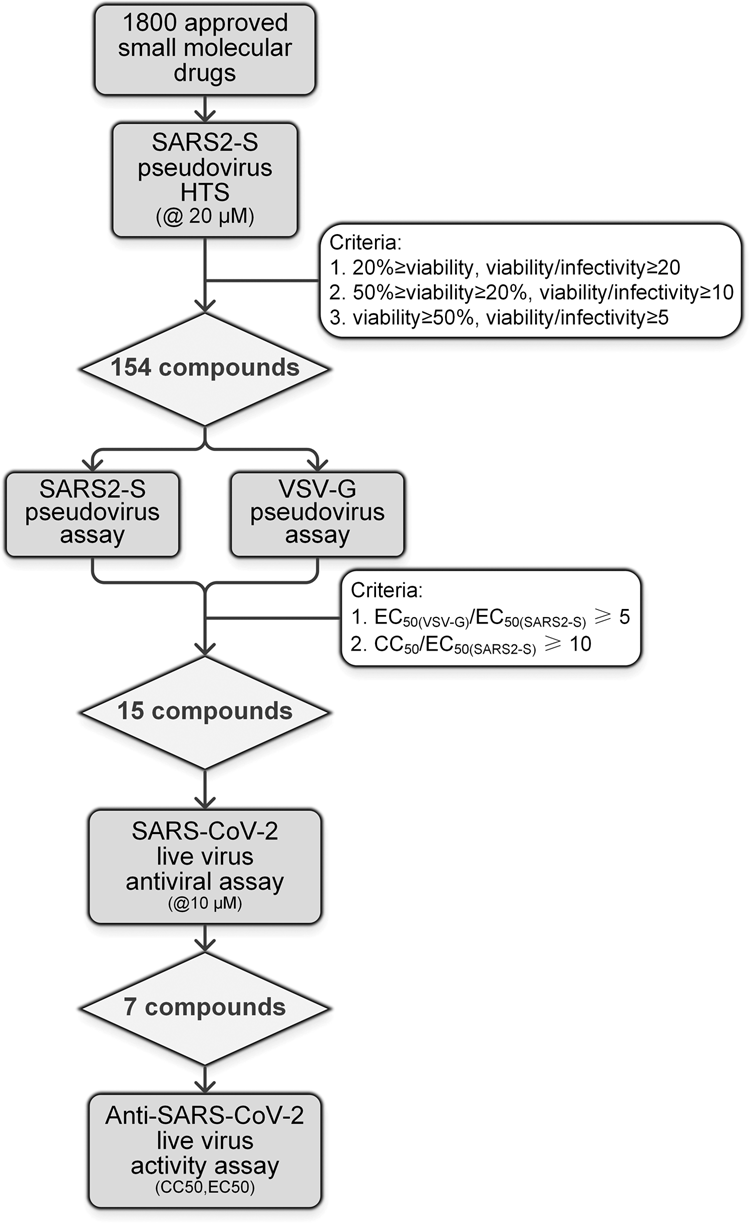
Identification Of Sars Cov 2 Entry Inhibitors Among Already Approved Drugs Acta Pharmacologica Sinica

Regulatory Process In The United States Of America Europe China And Japan Sciencedirect

Fda Update The Fda S New Drug Approval Process Development Premarket Applications

Fda Update The Fda S New Drug Approval Process Development Premarket Applications

Drugs Repurposed For Covid 19 By Virtual Screening Of 6 218 Drugs And Cell Based Assay Pnas

Sunscreen Products Rationale For Use Formulation Development And Regulatory Considerations Sciencedirect

Fda Update The Fda S New Drug Approval Process Development Premarket Applications
A Tool To Make Fda Drug Approval Practices Transparent Ucla Anderson Review

Fda Update The Fda S New Drug Approval Process Development Premarket Applications

Use Extension Studies To Enhance Phase 3 Data

Posting Komentar untuk "Why Is Blind Testing Used In The Drug Approval Process"